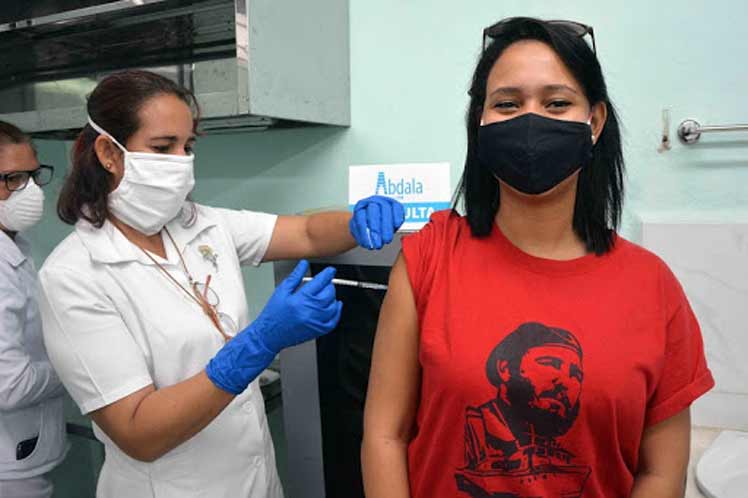
Havana, Cuba: While the Cuban anti-Covid-19 vaccine candidate Abdala is still under investigation to verify its effectiveness, his counterpart Soberana 02 today enters into the application of the third dose of his scheme of zero, 28 and 56 days, Prensa Latina publishes.
Abdala, developed by the Center for Genetic Engineering and Biotechnology (CIGB), closed on May 1 the vaccination cycle also of three doses, but in a short design, of zero, 14 and 28 days.
It is now necessary to evaluate the appearance of positive cases with symptomatic infection, its developers explained. In this way they will be able to compare the proportions between the vaccinated group and the one that received placebo.
Phase III of the clinical trial with Abdala began on Monday, March 22, in 20 clinical sites and 44 vaccination centers in the provinces of Santiago de Cuba, Guantánamo and Granma, cities that pass through a complex epidemiological scenario.
Meanwhile, Soberana 02, from the Finlay Vaccine Institute and the Island’s first candidate to enter phase III trials, will continue its research with the application of the third injection for those included in the two-dose schedule of the formulation and one of reinforcement, of Soberana Plus.
At the same time, in the capital, a population intervention study will be carried out starting today, which will include 1,700,000 people, especially in those municipalities not included in phase III of Sovereign 02.
The idea is to achieve immunization but in a staggered manner in risk groups, members of the scientific community explained at various times.
The vaccination strategy is in line with the requirements of the World Health Organization, to carry out studies parallel to the one carried out in phase III, provided that the safety of the candidate formulations is demonstrated.

Digital writing
Equipo de redactores del sitio web de Radio Mayabeque