Havana: Cuba today has two of its own anti-Covid-19 vaccine proposals whose efficacy results against the disease exceed the 50 percent limit recommended by the World Health Organization (WHO), Prensa Latina publishes.
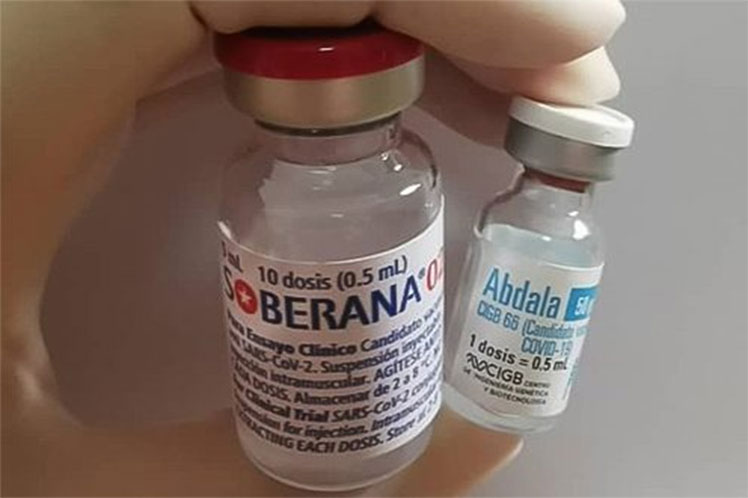
The day before, the Finlay Institute of Vaccines (IFV) announced that two doses of the vaccine candidate Soberana 02 plus an injection of its counterpart Soberana Plus showed 91.2 percent efficacy in terms of the ability to prevent symptomatic disease.
The general director of that entity, Vicente Vérez, assured that this high number could also be demonstrated against the strain identified for the first time in South Africa.
On June 18, IFV scientists confirmed that Soberana 02 with two injections was 62 percent effective, to which is added a booster dose of Soberana Plus.
For its part, the Center for Genetic Engineering and Biotechnology (CIGB) reported on June 21 the efficacy of the vaccine proposal Abdala with three doses, whose figure was 92.28.
Different research vaccination modalities have been carried out with both candidates, such as clinical trials, intervention studies and health intervention, during which a total of 6 million 833 thousand 720 doses have already been applied.
Currently, Cuba is awaiting authorization for emergency use of these vaccine candidates by national regulatory authorities that analyze the required documentation and inspect the production process.
National authorities assured on several occasions that this could be the first country in the world to have its entire population vaccinated against Covid-19 by the end of 2021 with its own candidates.
In addition to the three aforementioned proposals, this Caribbean nation has Sovereign 01, from the IFV; and Mambisa, from CIGB, who are progressing through different phases of clinical trials.
Data from the WHO and several specialized sites summarize that Pfizer and BioNTech, a vaccine developed by an American pharmaceutical company in conjunction with the German technology company, showed 95 percent efficacy.
Moderna, another proposal from the North American country reached 94.1; followed by the Russian Sputnik V with a percentage of 91.6 and the American Novavax with 90.
Chinese proposals such as Sinopharm and Sinovac show efficiency of 78 and 51 percent, respectively; while that of the American pharmaceutical company Johnson & Johnson, which only requires one dose, showed 66.9.
Likewise, the one developed by the University of Oxford and the British-Swedish pharmaceutical company AstraZeneca presented 63.09 percent.