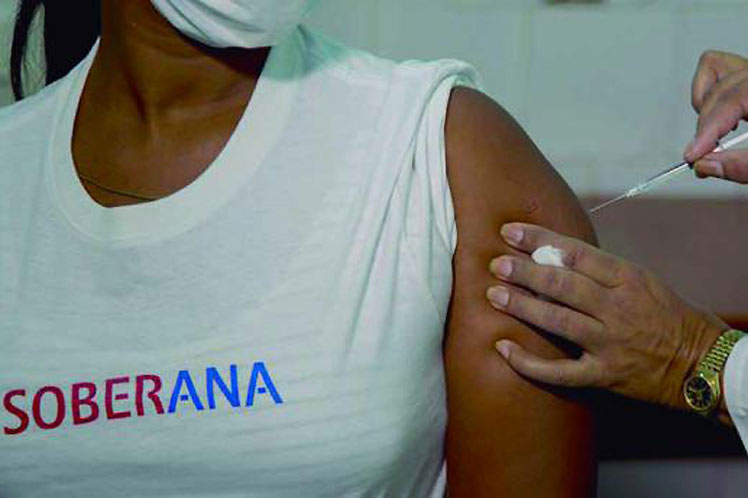
Havana: After a week of monitoring the first volunteers, the research team of the Cuban vaccine candidate against Covid-19 will deliver the files today to the regulatory entity that will authorize them to go up to the next step of the phase I clinical trial, Prensa Latina reports.
The first group made up of 20 individuals aged 19 to 59 years was vaccinated the previous Monday to assess the safety of the drug and, according to reports from its developers, all are in good health.
The only adverse effect is mild pain at the injection site, a common side event for all vaccines, early reports indicated.
After this step and everything normal as stipulated in the protocols, the Center for State Control of Medicines (Cecmed), the regulatory entity, will give the green light to the vaccination of the second planned group, made up of the same number of volunteers, but with ages of 60 to 80 years.
Named Soberana 01, the specific injectable drug against Covid-19, developed by the Finlay Institute of Vaccines (IFV), entered its first phase of clinical trials in humans after showing positive results in the preclinical stage (in animals).
This trial characterized as adaptive, multicenter, controlled and randomized, in the latter case it means that the 20 volunteers receive the injectable in two of its variants, a lower and a higher dose, or the control product: the Cuban VA-MENGOC meningococcal vaccine -BC, one of the IFV leaders.
As the research methodology indicates, in this case the study with Soberana 01 will be double blind, that is, neither the participating individuals nor the researchers know who belongs to the control or experimental group.
Since the arrival of the disease and after the sequencing of its genome, the Cuban scientific community has worked in the search for its own injectable and focused its studies on several proposals based on previous platforms.
The IFV carries out four projects as candidates for a specific vaccine against the disease that has hit the entire planet since the beginning of the year, and the first of them defined was named Finlay FR, Front Runner category.
Once its phase I has concluded, it will enter the next stage on September 11 with a sample size of 676 volunteers, including 40 from the initial period.
The research, which in all its stages should conclude in January 2021 and available to the population in February, joins the group of 30 that worldwide received authorization from the World Health Organization to advance in the clinical trial in humans.
The only one designed so far in Latin America and the Caribbean, the vaccine will not be the first to appear in the world, but it will be the one that will cover all Cubans. Our population will be the first vaccinated, it is a dream, the IFV director, Vicente Vérez, assured the multinational Telesur.