Havana, Cuba: Cuban scientists are about to complete the efficacy study of the Sovereign 02 vaccine candidate with the third booster dose today, to make this variable known definitively.
The data provided on the quality and quantity of antibodies generated with a third administration of this injectable constitutes the final step to present the research to the regulatory authority and validate it as a vaccine.
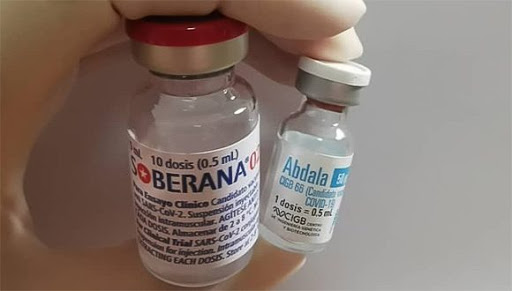
This is how its developers from the Finlay Vaccine Institute (IFV) explained, after announcing last Saturday that the candidate in his two-dose scheme achieved 62 percent effectiveness.
In phase III of the Soberana 02 clinical trial, its 44 thousand 10 volunteers were divided into three groups for the study, two experimental and another control with placebo.
One received two doses of the formulation at an interval of zero, 28 and 56 days, while the second received the same scheme plus a booster with Soberana Plus.
This is the first intermediate analysis of this variable, which compares the incidence of symptoms in placebos with respect to vaccinated subjects, the IFV research director Dagmar García in a recent intervention explained.
The candidate in phase I / II demonstrated safety and immunogenicity and in the later stage the analyzes focus on verifying its efficacy, that is, what effect it has on symptomatic disease, severe disease, infection and transmission.
Confident from the outset with this scientific idea taken to a bulb, the director of the IFV, Vicente Vérez, repeated several times with certainty that the project was safe.
The result is very comforting; it was obtained in a scenario of circulation of mutant strains of the virus. It is not an efficacy against the original strain, but against those that circulate today in Havana, worrying about its transmissibility, he said.
It is a result that exceeds that recommended by the World Health Organization of 50 percent efficacy for approval, said the scientist after explaining that the global health entity also stipulates that the confidence interval exceeds 30 percent, and in this case exceeds 40.
The conclusion of the phase III study could be known in two weeks. Now they are under evaluation, and we can predict that it will be higher than the 62 percent that we reach with two doses, the researchers predicted.
Abdala, the country’s second anti-Covid-19 proposal from the Center for Genetic Engineering and Biotechnology is heading these days to open codes to know the vaccinated and those of the control group to also evaluate efficacy.
Once Soberana 02 has been validated by the regulatory authority as a vaccine, it will advance to emergency vaccination, as stipulated in the protocols. The idea is to immunize 70 percent of the population by August.