Washington: Cuba’s commitment to self-produced COVID-19 vaccines is bearing fruit, the site https://www.nature.com highlighted today.
An article subscribed by Sara Reardon in Nature, a weekly international journal, with offices in several cities around the world, highlighted that preliminary data shows that a combination of three doses of the Soberana 02 vaccines is 92.4 percent effective in clinical trials, Prensa Latina publishes.
When the Covid-19 pandemic began, Cuba decided not to wait for the rest of the world to develop vaccines. The 60-year-old economic embargo (blockade) of the United States against the country, which prevents the export of products manufactured in the northern nation, would make it difficult to acquire drugs, researchers and officials knew, the article said.
“The best thing, to protect our population, was to be independent,” according to Vicente Vérez Bencomo, general director of the Finlay Vaccine Institute in Havana.
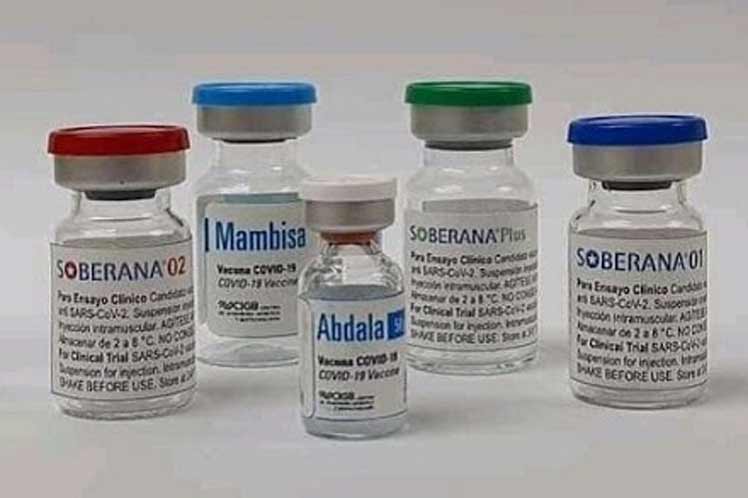
When evaluating the efficacy of Cuban immunogens, the publication highlighted the case of Soberana 02, from the Finlay Institute.
He added that “it appears to be effective against the highly transmissible Delta variant of the SARS-CoV-2 coronavirus, which has caused an increase in hospitalizations and deaths worldwide and now accounts for nearly all Covid-19 cases in Cuba”.
Nature addressed the work developed by Cuban scientists at the Center for Genetic Engineering and Biotechnology (CIGB) in Havana with Abdala, a three-dose vaccine, which was more than 92 percent effective in phase III trials that included more than 48 thousand participants, but the full results have not yet been published.
The journalist specified that in the studies carried out by the Finlay Institute vaccines reached “an efficacy similar to that of those manufactured by Johnson & Johnson (J&J) in New Brunswick, New Jersey, and AstraZeneca in Cambridge, United Kingdom” and directors of that center indicated that they can produce 10 million doses of Soberana 02 per month.
Protein-based vaccines such as Soberana 02 and Abdala may have some advantages over other types of injections, said Craig Laferrière, head of immunogen development at Novateur Ventures in Toronto, Canada, who has been comparing the safety and efficacy of the injections. of COVID-19.
Unlike the messenger RNA (mRNA) vaccines produced by Pfizer, based in New York, and Moderna, based in Cambridge, Mass., Proteins such as Cuban ones do not need to be kept at extremely low temperatures, which facilitates their distribution in remote areas.
Laferrière also estimated that the Cuban drug could “have fewer side effects than the AstraZeneca and J&J vaccines, which use an adenovirus to introduce the gene for a different portion of RBD into cells and have been linked to blood clots.”
In assessing how protein-based Covid-19 vaccines could change the pandemic, John Grabenstein, president of the consultancy Vaccine Dynamics in Easton, Maryland, noted that he believes “it will be a useful addition to the world.”
Meanwhile, he pointed out the Nature article, Cuba continues with its strategy to develop the vaccine against Covid-19, in addition to those mentioned, the Sovereign 01 from Finlay, and the Mambisa from the CIGB, which are in the clinical trial phase.

Redacción Digital
Equipo de redactores del sitio web de Radio Mayabeque